Your Partner for Medical Device Development
Archimedic helps companies reduce risk and accelerate time to market for new medical devices.
100% Focused in MedTech
Since 2009, Archimedic has been helping companies develop drug delivery, surgical, diagnostic, and home health medical devices. Our work spans regulatory strategy, concept development, industrial design, human factors, prototyping, production engineering, design control, V&V, and manufacturing transfer.




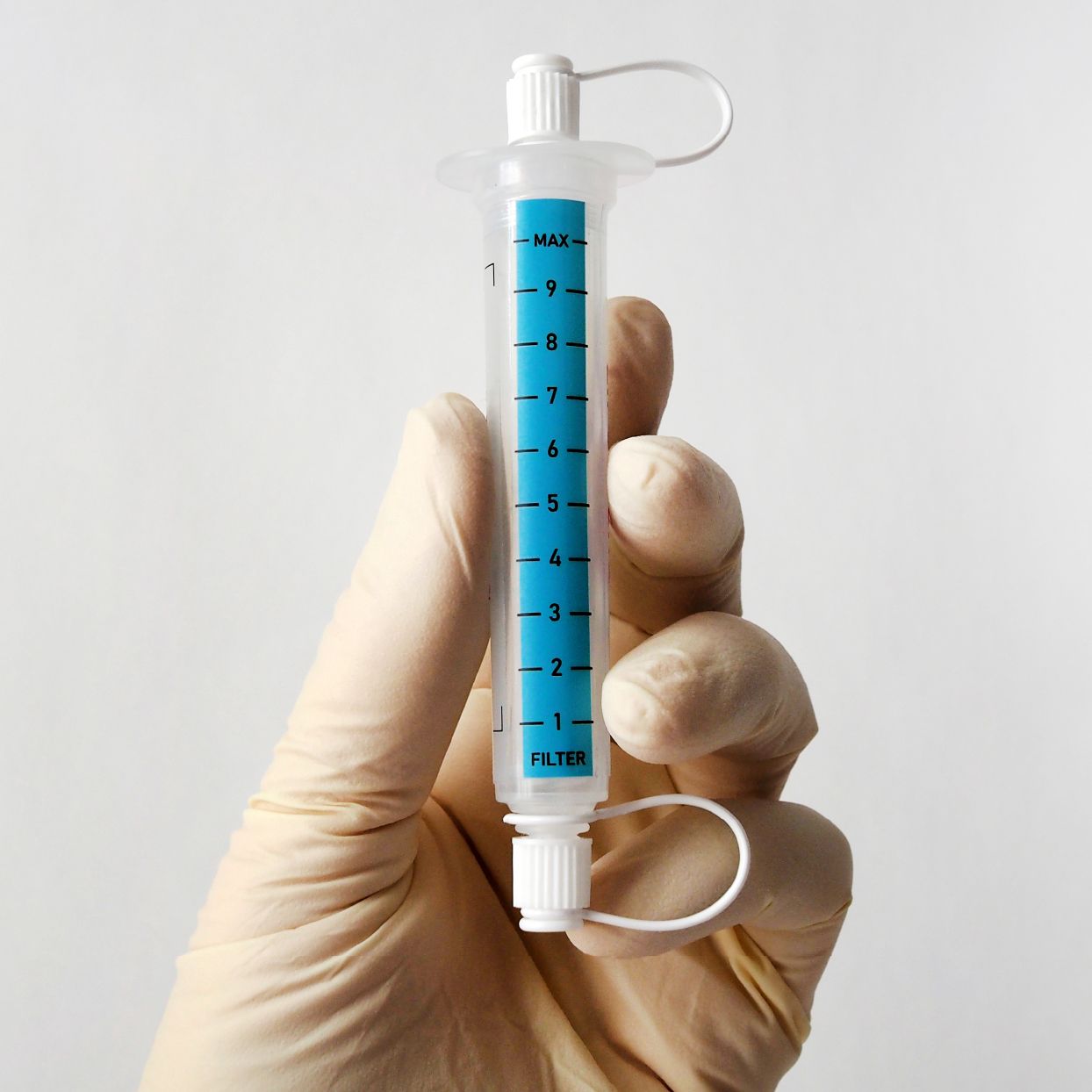
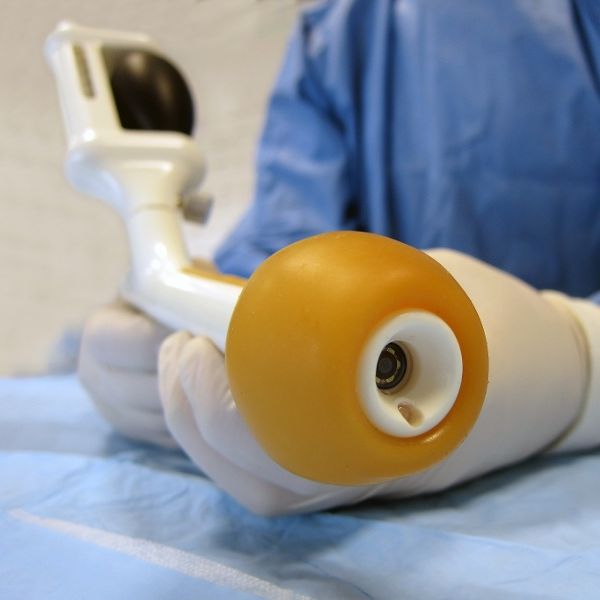
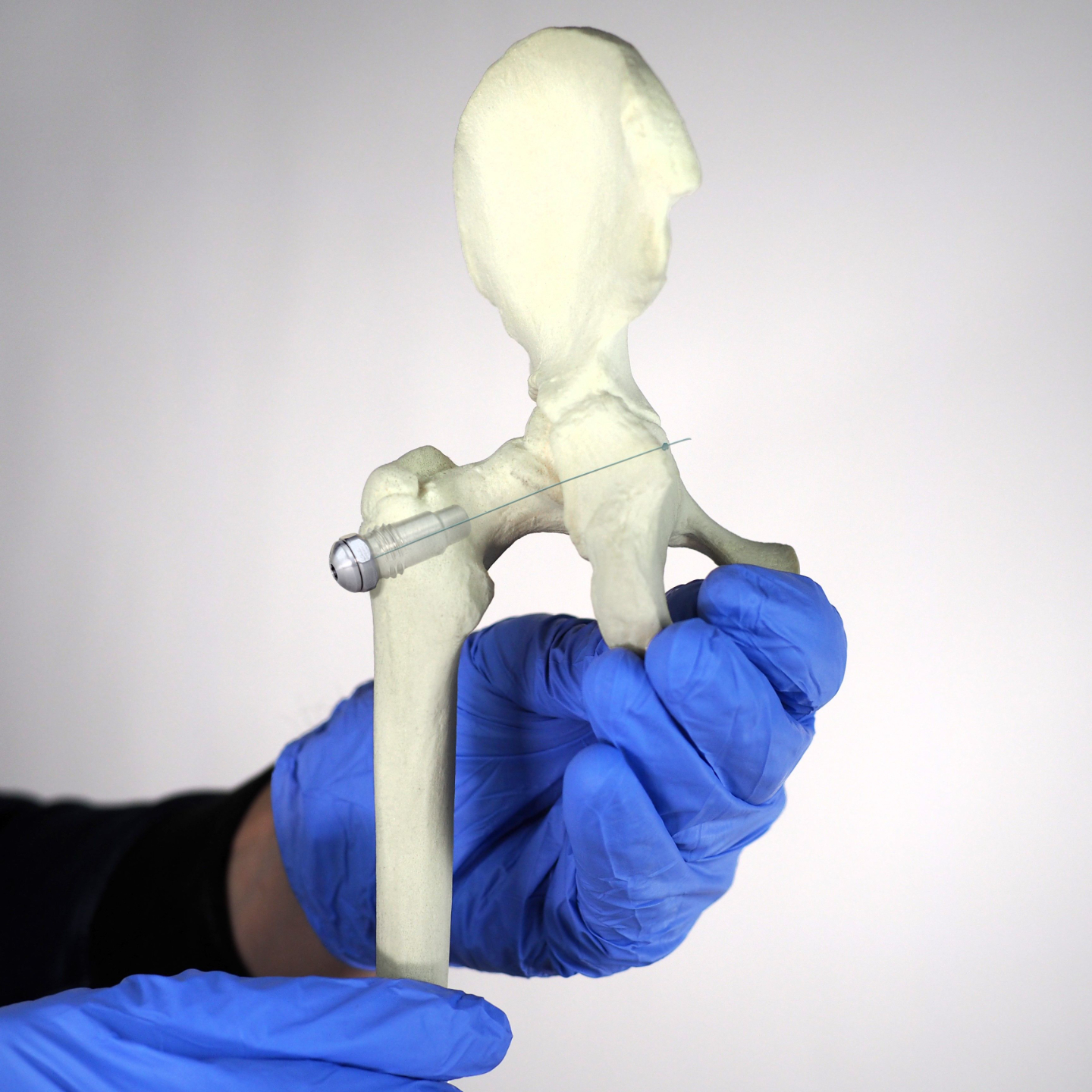



Plug-and-play MedTech expertise to accelerate your device development
At Archimedic, we provide drug delivery expertise and an ISO 13485 certified QMS to help companies transform concepts into reliable medical devices.
> About ArchimedicWhat do our clients say?
Wondering if we can help you Advance your MedTech Venture?
Set up a no-pressure intro session to explore possibilities together.
> Let's ConnectHow we support MedTech
Our programs are custom tailored to your unique requirements, but below are some of the ways we can help advance your MedTech program.
Regulatory
Standards and predicate research, pathway definition, indications for use statements, product labeling, pre-submission (Q-Sub) meetings, regulatory submissions

Quality
Requirements definition, risk management, design control, documentation control, design history file (DHF) management, device master record (DMR) generation.
Human Factors
Task mapping, heuristic evaluations, formative studies, user related risk analyses (URRA), instructions for use (IFU), threshold analyses, summative studies, usability file.

Engineering
Concept development, mechanism design, electromechanical design, plastic part design, finite element analysis (FEA), design for manufacturability & assembly (DFMA).

Industrial Design
User experience design, ergonomic modeling, aesthetic development, brand integration, usability mockups, visual models, color-material-finish specification.

Prototyping
Mechanism prototypes, electrical breadboards, ergonomic mockups, Alpha/beta prototypes, packaging prototypes, rapid tooling samples.

Testing
Feasibility testing, test method and protocol development, characterization testing, gage R&R, design verification testing (DVT), external test house management, test reporting.

Manufacturing
Key supplier research and auditing, supply chain development, quoting, design transfer, first article inspections, process debugging, clinical unit builds, pilot runs.

Program Management
Team alignment, issues resolution, action item management, timeline and budget tracking, meeting facilitation, external vendor management.
Need a Partner to Raise Funding and Execute?
Explore equity-based partnership through our venture studio model.
> MedTech Venture StudioOur Perspectives on MedTech
We don't deny it -- our views are different than convention. Get our take on the MedTech Mindset blog. Here are some of our recent posts.
Ready to explore possibilities?
Share your info in the form below for a no-pressure introductory call with one of our senior team members.




