MedTech Design Services
Designing Medical Devices that Change Lives
At Archimedic, Design is never just about how a device looks or functions.
Anyone can sketch a concept — the real challenge is designing a device that’s safe, manufacturable, and built to succeed in regulatory review and the marketplace.
Our team integrates engineering, human factors, quality, and regulatory insight from the start to ensure your innovation doesn’t just work — it wins approval and market adoption.
Schedule a Design Consultation
Our Core Design Capabilities
Design is where innovation takes form — but great Design doesn’t happen by accident.
At Archimedic, we follow a structured yet flexible approach that accelerates development, mitigates risk, and aligns every design decision with user needs, regulatory expectations, and manufacturing realities. Our capabilities span the full design spectrum, ensuring that every element of your device — from look and feel to function and feasibility — is built for success.
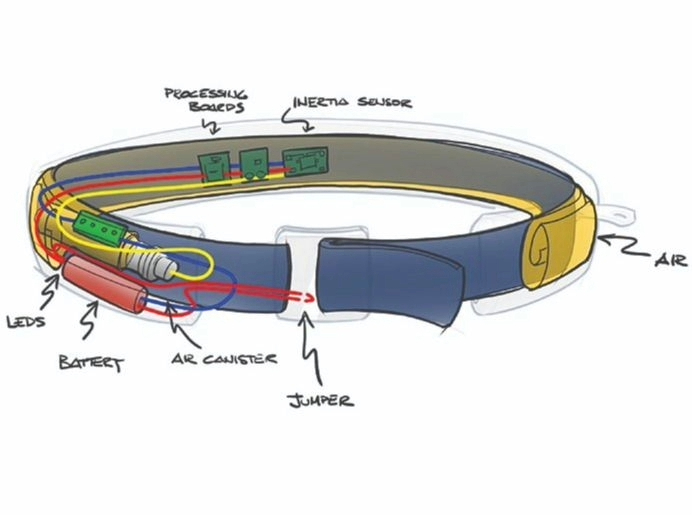
Concept Development & Prototyping
We expand early ideas into multiple design concepts, then de-risk them through breadboards, mockups, and prototypes. This rapid iteration uncovers challenges before they become costly.
Design for Manufacturability & Assembly
We design with scalability in mind — ensuring compatibility with materials, processes, and tolerance thresholds, we reduce surprises when your device moves into manufacturing.
Engineering Verification Testing (EVT)
Before design freeze, we build EVT units and confirm performance against critical inputs — verifying that the design is robust, reliable, and ready for validation and regulatory review.
Industrial Design & UX/UI
We merge visual design, usability, and human factors to craft devices that not only look right but feel right — intuitive, trusted, and adopted by users, driving confidence and differentiation in the market.
Human Factors Engineering & Testing
Through formative studies, we evaluate usability, identify workflow challenges, and surface potential risks — insights that lead to safer, more effective design and strengthen the foundation for regulatory validation.
Design Research & Strategy
Every successful design starts with understanding users — their goals, limitations, and needs. We translate these insights into structured design inputs that guide functionality, usability, and risk mitigation throughout development.
Verification & Validation (V&V) Planning
We map out the V&V activities needed to demonstrates safety and effectiveness, ensuring alignment with regulatory expectations, industry standards, compliance testing, risk management, and design controls.
Branding & Aesthetics
A device’s look and feel are critical to trust, adoption, and brand alignment. We translate your company’s identity into cohesive visual and tactile cues, ensuring the product communicates reliability, professionalism, and care from first glance to daily use.
Designing for Regulatory Approval & Adoption
Most MedTech firms stop at design handoff. Archimedic takes it further — integrating regulatory and go-to-market strategy into every phase of development. This means your device is engineered not just to function, but to meet FDA expectations and thrive in the hands of users and buyers.
Our integrated approach ensures that regulatory and go-to-market strategy are built into the foundation of every design — not added as afterthoughts:
- Quality & Regulatory Documentation – We support the development of your design history file (DHF) and device master record (DMR) in alignment with FDA requirements.
- Go-to-Market Alignment – Every design decision considers reimbursement models, cost-to-manufacture, and competitive positioning, so your device is both viable and valuable.
Why Choose Archimedic?
We’re more than your typical medical device firm.
We’re a full-path MedTech partner who understands that medical device success requires integration across Design, Regulatory, and Go-to-market workflows.
- Decades of experience navigating FDA and regulatory pathways for medical devices and combination products.
- Integrated DRG approach that embeds regulatory thinking into design and development.
- Proven track record of successful submissions across device classes and therapeutic areas.
- Collaborative team of engineers, strategists, and regulatory experts working under one roof.
- Flexible engagement models tailored to startups, scale-ups, and established MedTech leaders.
Let’s Design the Future Together
The right design partner can mean the difference between an idea that stays on the shelf and a device that changes lives.
At Archimedic, MedTech lives here. Let’s create something extraordinary together.
Schedule a 30-minute meeting with a senior member of the Archimedic team to explore possibilities of working together.
Schedule a Design Consultation
