MedTech Go-to-Market Services
Defining the Right Pathway to Market
At Archimedic, your Go-to-Market Strategy isn’t an endpoint — it’s where success begins.
Even the best-designed devices can stall without a clear path to approval, adoption, and reimbursement. That’s why we integrate Go-to-Market thinking from day one — aligning design, regulatory, and commercial strategies into a single, actionable plan.
Our team combines stakeholder research, clinical insight, and market expertise to define the right pathway for your device — from regulatory approval to real-world adoption.
Schedule a 30-Minute Consultation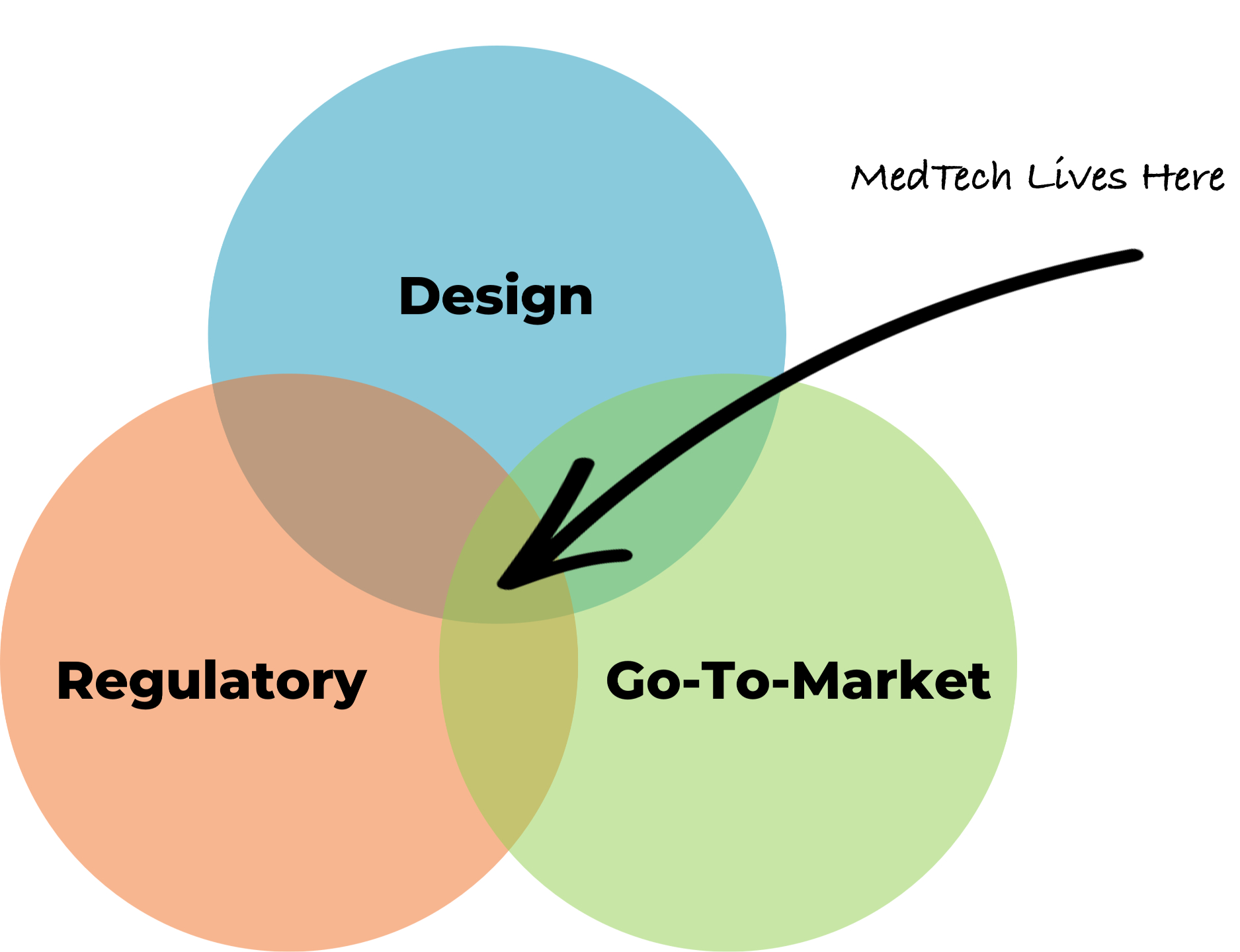
Our Core Go-to-Market Capabilities
A strong Go-to-Market strategy bridges innovation, regulation, and commercialization
At Archimedic, we develop integrated strategies that connect design, clinical evidence, reimbursement, and market adoption into one cohesive plan. Our Go-to-Market capabilities ensure your medical device doesn’t just clear regulatory hurdles — it achieves real-world success.
Regulatory Alignment
We research product codes, predicates, and standards to define a regulatory strategy that supports your market objectives and claims. Early alignment between regulatory and marketing pathways reduces risk and accelerates approval.
Market Positioning & Claims Strategy
We develop claims that resonate with clinicians, patients, and payers — and ensure they’re supported by appropriate evidence. This strategic balance between differentiation and compliance drives faster market adoption.
Clinical & Evidence Planning
We outline pre-clinical and clinical strategies to generate the evidence needed for both regulatory approval and commercial credibility. Our team aligns study design, endpoints, and data collection with desired claims and payer requirements.
Reimbursement & Payer Strategy
We analyze reimbursement codes, institutional purchasing pathways, and direct-to-consumer opportunities. By identifying viable reimbursement pathways early, we strengthen your business case and adoption potential.
Intellectual Property (IP) Strategy
We evaluate prior art and patent landscapes to help secure IP protection and identify white space for innovation. IP strategy is integrated with regulatory and marketing goals to support both defensibility and differentiation.
Business Model Development
We craft business models that align customer value with commercial viability. Whether through direct sales, partnerships, or licensing, we define structures that maximize your device’s reach and return.
Gaining Market Understanding Through Research & Discovery
Every successful medical device begins with understanding the patients, providers, and payers who shape adoption. At Archimedic, we combine research-driven insight with executional strategy to translate market understanding into action.
Through primary research, secondary data analysis, and expert consultation, we uncover the clinical, commercial, and behavioral drivers that define your opportunity. From there, we develop a comprehensive go-to-market roadmap that integrates technical, clinical, regulatory, and commercial milestones — ensuring your device moves forward with alignment, adoption, and impact.
Why Choose Archimedic?
We’re more than your typical medical device firm.
We’re a full-path MedTech partner who understands that medical device success requires integration across Design, Regulatory, and Go-to-market workflows.
- Decades of experience navigating FDA and regulatory pathways for medical devices and combination products.
- Integrated DRG approach that embeds regulatory thinking into design and development.
- Proven track record of successful submissions across device classes and therapeutic areas.
- Collaborative team of engineers, strategists, and regulatory experts working under one roof.
- Flexible engagement models tailored to startups, scale-ups, and established MedTech leaders.
Let’s Define Your Path to Market
The sooner you establish the right Go-to-market strategy, the greater your chance of success.
At Archimedic, MedTech lives here. Let’s build a plan that ensures your device doesn’t just get developed — it gets adopted in the market.
Schedule a 30-minute meeting with a senior member of the Archimedic team to explore possibilities of working together.
Schedule a 30-Minute Consultation