About Archimedic
We are a Focused Team of Medical Device Developers
Who We Are
Archimedic is a focused team of researchers, strategists, designers, and engineers that are committed to helping innovators create life changing medical devices and successful business ventures. Just like the Stomachion puzzle pieces in our logo, we will work with you to assemble a team uniquely positioned to solve the med tech challenges that you face.

Leadership in MedTech
LEADERSHIP TEAM

CHIEF EXECUTIVE OFFICER
M. Alexander Shaw, PhD
Alex leads operations, human resources, and strategic direction at Archimedic.

CHIEF INNOVATION OFFICER
Aaron Pavkov
Aaron leads the delivery of Archimedic's engineering services, interfacing directly with clients and ensuring a smooth journey through the engineering process.

VICE PRESIDENT, CLIENT ENGAGEMENT
Rightley McConnell
Rightley leads operations related to new business development and ongoing client relations.

FOUNDER & PRINCIPAL CONSULTANT
Eric Sugalski
Eric leads the technical team and is responsible for excellence in device development work at Archimedic.
Where We Work
A PLACE FOR TEAMWORK
Our office is located in beautiful West Chester, PA in the suburbs of Philadelphia. Our team works onsite in an inspirational, collaborative, and high-tech setting within a vibrant community.




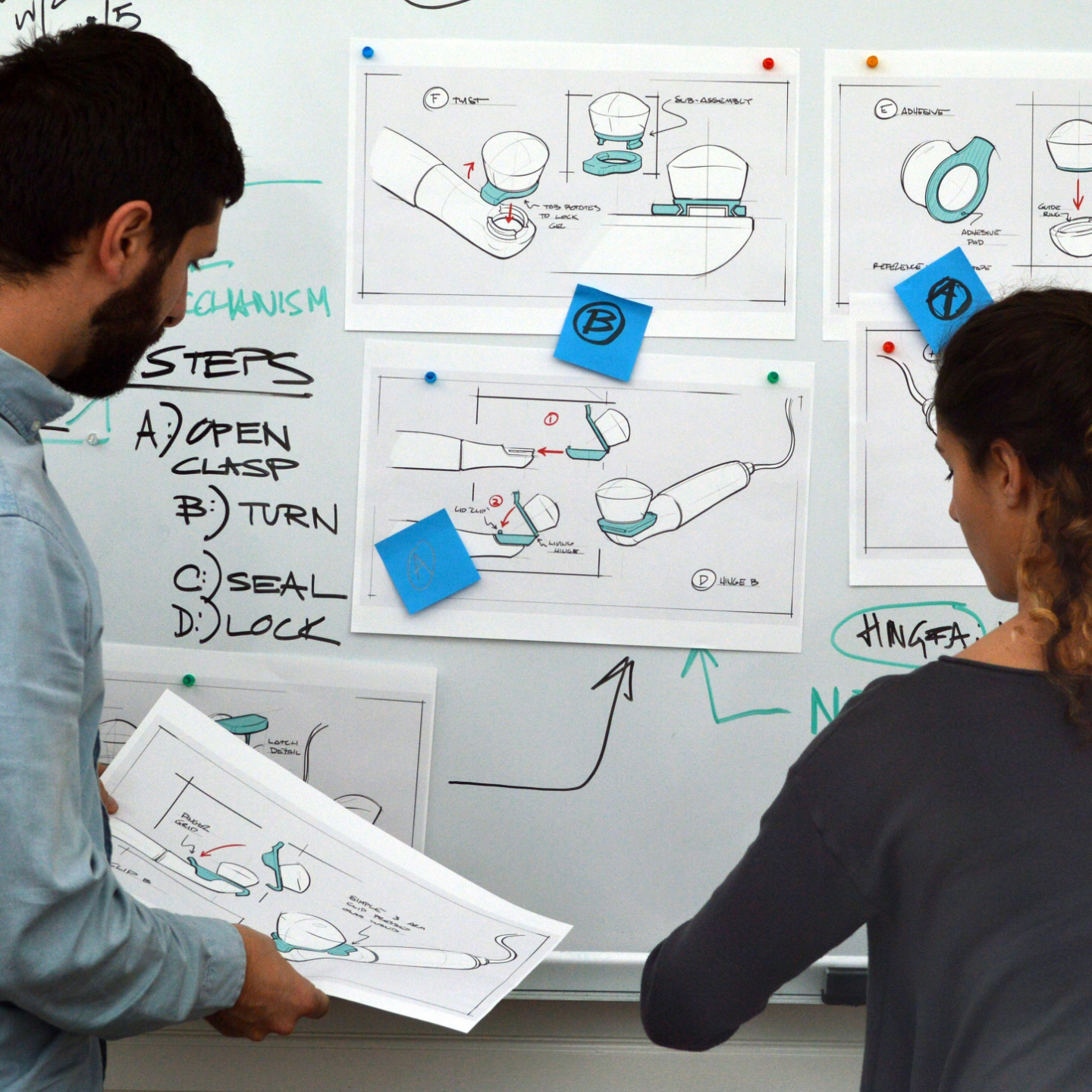
Our Services
We provide end-to-end medical device development support across Design, Regulatory, and Go-to-Market pathways.

Engineering
We can help you with R&D, prototyping, design for manufacturability & assembly, and reliability engineering.

Human Factors
We can help you with user-focused design, evaluation through formative studies, and summative validations.

Testing
We can help you with test protocols, data collection, and reporting from feasibility through V&V.

Quality
We can help you with requirements definition, risk management, design control, and full DHF management.

Regulatory
We can help you with pathway and strategy, management of pre-subs, and supporting regulatory submissions.

Program Management
Sounds basic, but one of the most important services we provide is to run point on critical programs.
Client Testimonials
What Our Clients Are Saying About Their Experience With Archimedic
Careers at Archimedic
Interested in Joining Our Team?
Head over to our Careers page to learn what we are all about and to explore career opportunities.
Careers at Archimedic