Partner with Archimedic to streamline design, secure regulatory authorization, and drive market adoption.
Where Innovation Meets Impact
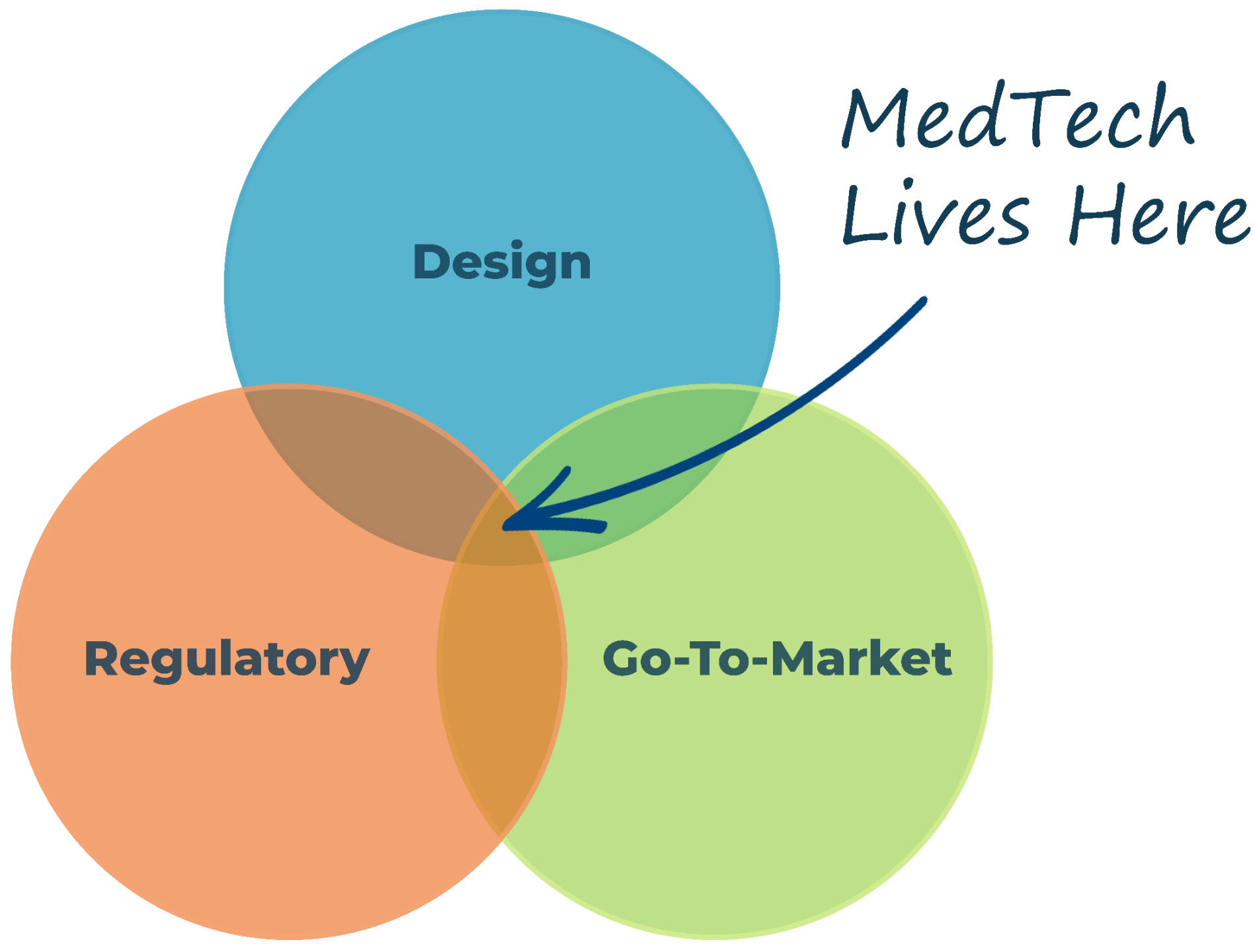
We go beyond great design to align your device with regulatory pathways and market needs, ensuring it thrives.
How We're Different: Watch the VideoMEDTECH MADE REAL
Case Studies
Learn how Archimedic has helped other MedTech innovators across surgical, drug delivery, diagnostic, and home health categories.
Open QMS
Elevate your quality standards with our open-source QMS designed to foster alignment and efficiency across Design, Regulatory, and Go-To-Market domains.
Design
Regulatory
Go-To-Market
LEVEL-UP IN MEDTECH
Blog: MedTech Insights
Dive into our blog for expert articles, podcasts, and resources that guide you through the complexities of MedTech-Market fit.
Impact Begins with Action
Your mission is clear. Let's connect to shape your device for market success and meaningful change.
Discuss My Project