MINIMIZING OPEN HEART SURGERY IN PEDIATRIC CARE
PEDIATRIC PERICARDIAL ACCESS PORT
PeriCor LLC is a spinout from Children’s National Hospital with a mission to eliminate the need for open cavity surgeries in pediatric patients requiring pacemakers and Implantable Cardioverter Defibrillators (ICDs). Aware of the significant risks and recovery burdens posed by traditional surgical methods, the founding team approached Archimedic to help develop a minimally invasive solution tailored to the unique anatomical and clinical needs of children, with potential use in adult populations. To bring this concept to market, PeriCor and Archimedic collaborated through the full development of a laparoscopic surgical port, from initial concept through manufacturing and commercialization.


AN INTEGRATED DESIGN APPROACH
User Needs Driven Design
Archimedic began by working with PeriCor to capture detailed user needs based on the clinical realities of pediatric cardiac surgery. These needs were translated into formal design inputs that addressed anatomical constraints, procedural workflows, and safety considerations. From there, the team defined clear design outputs that aligned with manufacturing feasibility and regulatory expectations. This structured approach ensured that PeriCor’s design history file was built on a solid foundation, giving the mechanical engineering team precise requirements to guide the design and development of the device so that it could meet user needs and the demands of real-world surgical use.
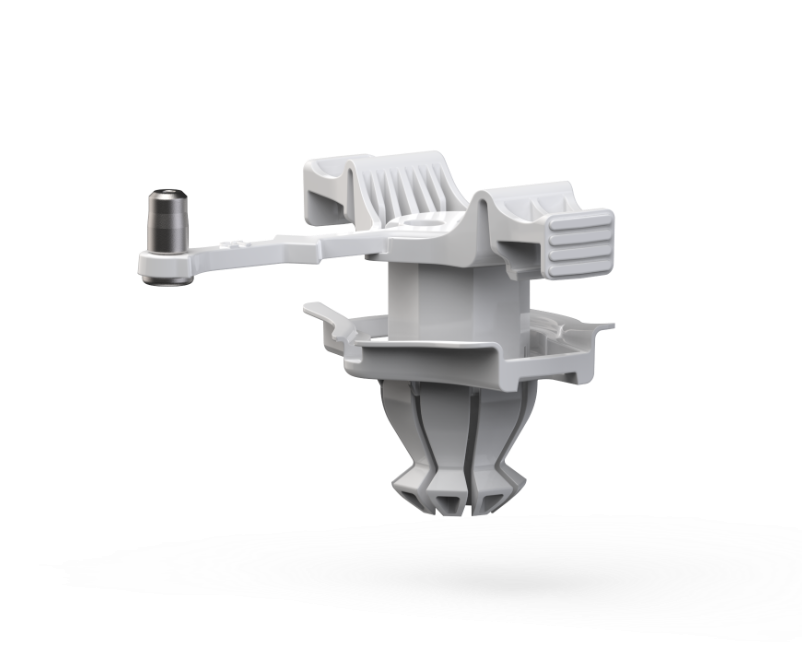

DESIGN-BUILD-TEST
Prototyping & Testing
Archimedic led the prototyping and testing process, guiding PeriCor through a structured build-test cycle that translated design outputs into functional models. In close collaboration with the client, Archimedic managed each iteration by constructing prototypes, conducting simulated surgical evaluations, and refining features based on performance data and clinical feedback. This method ensured that every design decision was based on real-world use and that PeriCor’s device progressed with clarity, precision, and validated confidence.
SUPPORTING CLINICAL RESEARCH
Manufacturing & Validation
In the next phase of development, Archimedic led the transition from prototype to production-ready design, establishing the documentation, inspection protocols, and material specifications required for regulatory submission and approval. The first step was vendor selection, where Archimedic leveraged its years of partnership with established injection molders. With a qualified contract manufacturer in place, Archimedic developed a mold-ready design that maximized quality while minimizing manufacturing costs. Once mold samples met engineering confidence testing, a pilot production run was manufactured to support all the validations necessary for market introduction: biocompatibility, functionality, packaging, and sterility. This phase marked a critical shift from development to deployment, with Archimedic providing the technical leadership needed to move the device into active research settings and real-world surgical application.

Ready to discuss your project?
Click the link below to learn how we start the conversation and explore collaborations.
GET STARTED