Speed versus Scale in Contract Manufacturing
Jun 15, 2022
What is your manufacturing strategy? There are several options to consider. Sometimes it makes sense to engage a large CMO early, whereas other times it makes sense to launch manufacturing with a smaller CDMO that also supports product development. The right manufacturing strategy is not one-size-fits-all. This article aims to explore the different medical device manufacturing strategies and the pros and cons to each.
But first, some definitions:
- Large CMO - A large Contract Manufacturing Organization (CMO) is typically a global operation that has thousands of employees. They build supply chains and manufacture devices typically ranging from 10 thousand to 10 million units/year, depending on the device complexity. Examples of large CMOs include Flextronics, Jabil, and Plexus.
- Small CDMO - A small Contract Development and Manufacturing Organization (CDMO) is an operation that typically has less than 200 employees. They focus on design and development activities on the front end, often extending services into early stage manufacturing. Examples of small CDMOs include Starfish Medical, Ximedica, and Archimedic.
Ultimately, most medical device companies need to transition to a large CMO for full-scale manufacturing. It's not a question of "if" -- it's a question of "when." A large CMO can help a medical device company scale and reduce costs in ways that smaller CDMOs simply cannot due to size constraints.
When considering the shift to a large CMO, it is helpful to think about the stages of manufacturing. Many companies use the following stages and definitions:
- Full Scale - The stage when manufacturing output matches the expected market demand after a product has gained traction. Volumes are typically in the hundreds of thousands or millions of units per year.
- Pilot - The initial commercial launch of saleable products following regulatory clearance. Volumes are typically in the low thousands. Pilot is a temporary stage with intentions of migrating into full scale production after manufacturing processes have been fine-tuned.
- DVT - DVT stands for Design Verification Testing. This is the formal verification process where design outputs are evaluated with respect to design inputs. This testing may include electrical safety testing, lifecycle testing, and force testing. DVT units are also typically used to conduct validation work, including human factors validation, biocompatibility validation, and clinical validation. DVT volumes are typically in the hundreds to low thousands.
- EVT - EVT stands for Engineering Verification Testing. This is typically pre-testing using prototype runs to evaluate basic functionality before design freeze. Similar tests are conducted in EVT and DVT, but EVT is generally lower volume testing with less extensive reporting. EVT volumes are typically in the tens to hundreds range.
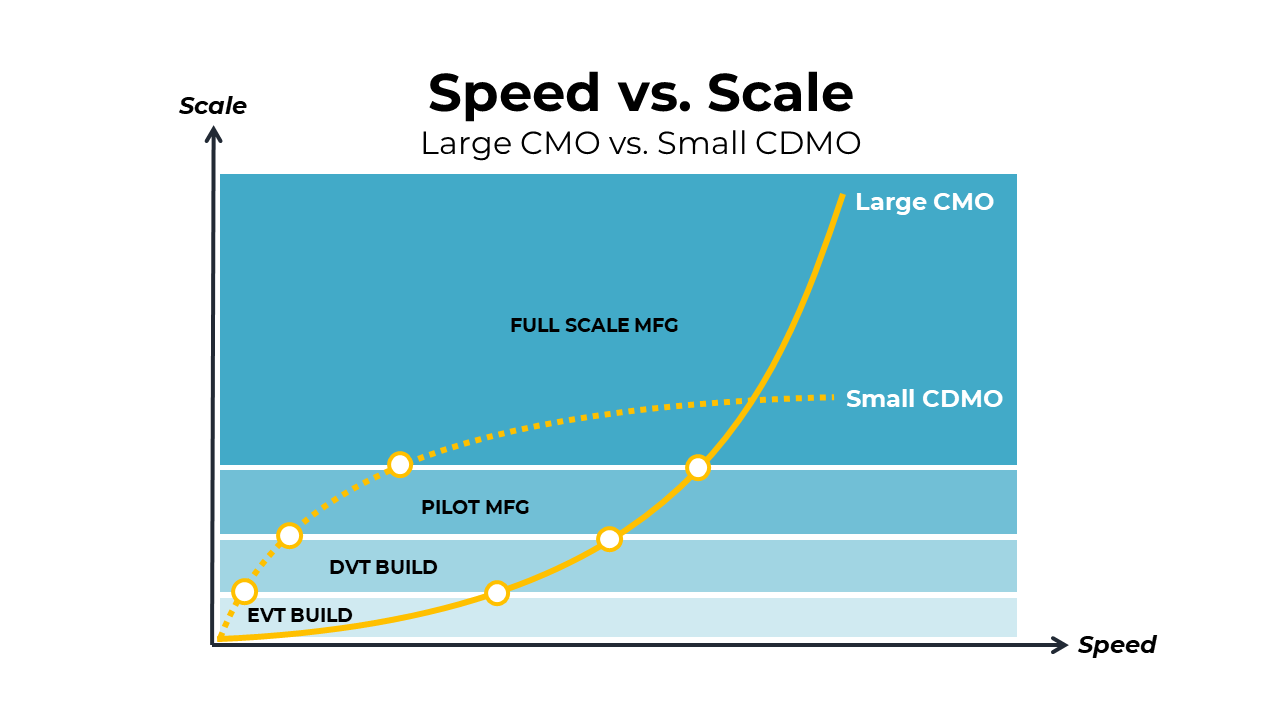
The graphic below illustrates the punchline.
Large CMOs can hit the scale, but ramp-up time can be long. Small CDMOs, on the other hand, can hit early milestones much more quickly, but their volumes will cap out at a relatively low quantity in comparison to large CMOs.
So, the question that most medical device companies have is -- when is the optimal time to transition from a small CDMO to a large CMO? Below are a few scenarios to help with this critical decision.
Transitioning after Pilot
Many companies engage a small CDMO to accelerate the completion of EVT, DVT, and Pilot runs. While the pilot units are being distributed and trialed, a larger CMO is establishing and validating the full scale production lines. This strategy gets to market rapidly and provides a manufacturing bridge during low volume requirements, until the higher volume demand is required. This strategy typically works well for high-cost, low volume devices, such as point-of-care diagnostics, robotic surgical systems, imaging systems, and other capital medical equipment. The graphic below illustrates this scenario of transitioning to a large CMO after pilot.
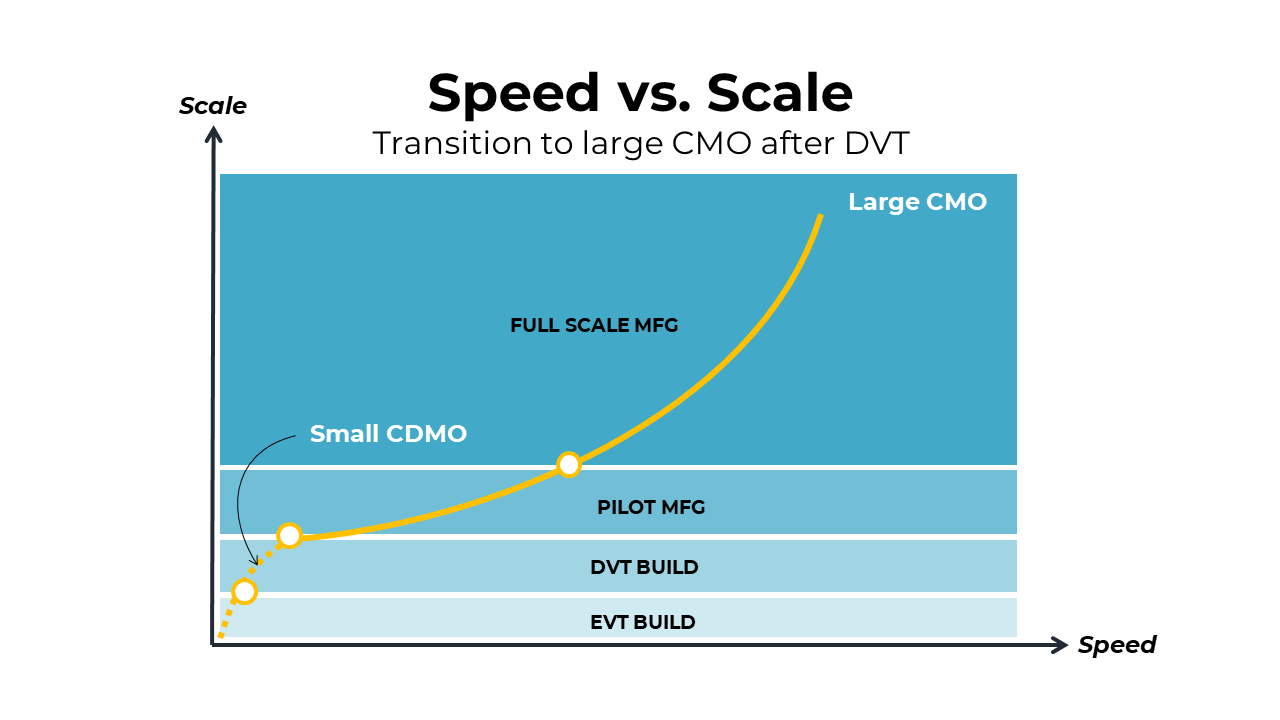
Transitioning after DVT
This next scenario illustrates transitioning to a large CMO after the DVT build has taken place. The large CMO would then be responsible for launching pilot and flowing directly into full-scale manufacturing. This may be a cleaner route into manufacturing with fewer process validations, but the tradeoff is speed in producing the pilot units. The large CMO will require much more time to establish the pilot line than it would take for a small CDMO to do the same. This strategy may be appropriate for mid-volume electromechanical medical devices, such as at-home therapeutic devices, wearable monitoring devices, and in-hospital non-invasive diagnostics. The graphic below illustrates this transition to a large CMO after DVT.
Transitioning after EVT
The following scenario is where the transition to a large CMO occurs right after EVT. This route avoids redundancy of process validations, such as installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). These process validation steps are required for organizations completing DVT and pilot units, so the scenarios above include IQ-OQ-PQ redundancies between a small CDMO and a large CMO. In the case of very high-volume devices (1M+ units/year), this process often makes the most sense. In order for these types of high-volume devices to be economically viable, there may need to be automation systems or low-cost labor resources that are providing the necessary scale. This scenario is often the case for drug delivery devices. The graphic below illustrates the scenario of transitioning to a large CMO after the EVT build.
Summary
The appropriate time to transition from a small CDMO to a large CMO is a strategic decision. It depends on the criticality of speed to achieving early milestones, your confidence in the time horizon to scale, and the unit costs required for commercial viability. In any of these scenarios, it is important to think through the manufacturing partnerships you will need and start these conversations early. The ramp-up times may take much longer than you think, and it will be important to embed realistic timelines into your commercial strategy from the outset.
Support for MedTech at Every Stage
Archimedic partners with medical device teams to solve complex design, development, regulatory, and go-to-market challenges.